The Ethics Committee for Research Involving Human Subjects Universiti Putra Malaysia (Jawatankuasa Etika Universiti Penyelidikan Melibatkan Manusia) started as The Medical Research Ethics Committee. This committee included the Animal Ethics component in 1998. Recently, however, the Animal Ethics Committee has been made as an independent entity dealing only with animal care and use under the official name of IACUC (Institutional Animal Care and Use Committee).
The Ethics Committee for Research Involving Human Subjects (more commonly referred to with its Malay acronym, JKEUPM) was established under the authority of the Senate of Universiti Putra Malaysia on 8 September 2011. JKEUPM is specifically given the task of protecting research participants, and to make researchers be responsible in ensuring that the basic principles regarding the use of human subjects are observed in their research. JKEUPM is guided in its stance and decisions by the principles expressed in the Declaration of Helsinki (2008). The Declaration, which was first developed by the World Medical Association (WMA) in 1964, was intended to outline a number of ethical principles that need to be adhered to when medical research involving human subjects is carried out. There are numerous principles listed in the Declaration, but it states above all that the well-being of the human subject of the research must take precedence over all other interests and regulations, including national and international regulatory requirements.
In addition to the Declaration, a number of provisions from the Nuremberg Code of 1946 which pertain to the gathering of information in social science fields have also been utilised in the present set of guidelines, particularly the emphasis on protecting a subject’s privacy, accurately representing the aims of a particular study to a subject, and the necessity of safeguarding a subject’s well-being by not deliberately placing him/her in situations that can be deemed compromising.
JKEUPM is also guided by the National and International Ethical Guidelines for Biomedical Research Involving Human Subjects (CIOMS). JKEUPM recognizes ethical clearance from other Ethics Committees, e.g. from the Ministry of Health and other universities, which are recognized by the National Pharmaceutical Control Bureau (BPFK), Ministry of Health Malaysia. Thus, a research project which has received ethical clearance from any of these other committees does not require a separate clearance from JKEUPM. However, if the research project involves either undergraduate or postgraduate students at any point, a JKEUPM ethical clearance is needed. The JKEUPM seeks to fulfil the requirements for international assurances and is established and functions in accordance with Malaysian law and regulations.
The JKEUPM has the authority to:
a. Approve, disapprove or modify studies based upon consideration of aspects related to human subject protection;
b. Request progress reports from investigators and oversee the conduct of the study;
c. Suspend or terminate the approval of a study; and
d. Place restrictions on a study.
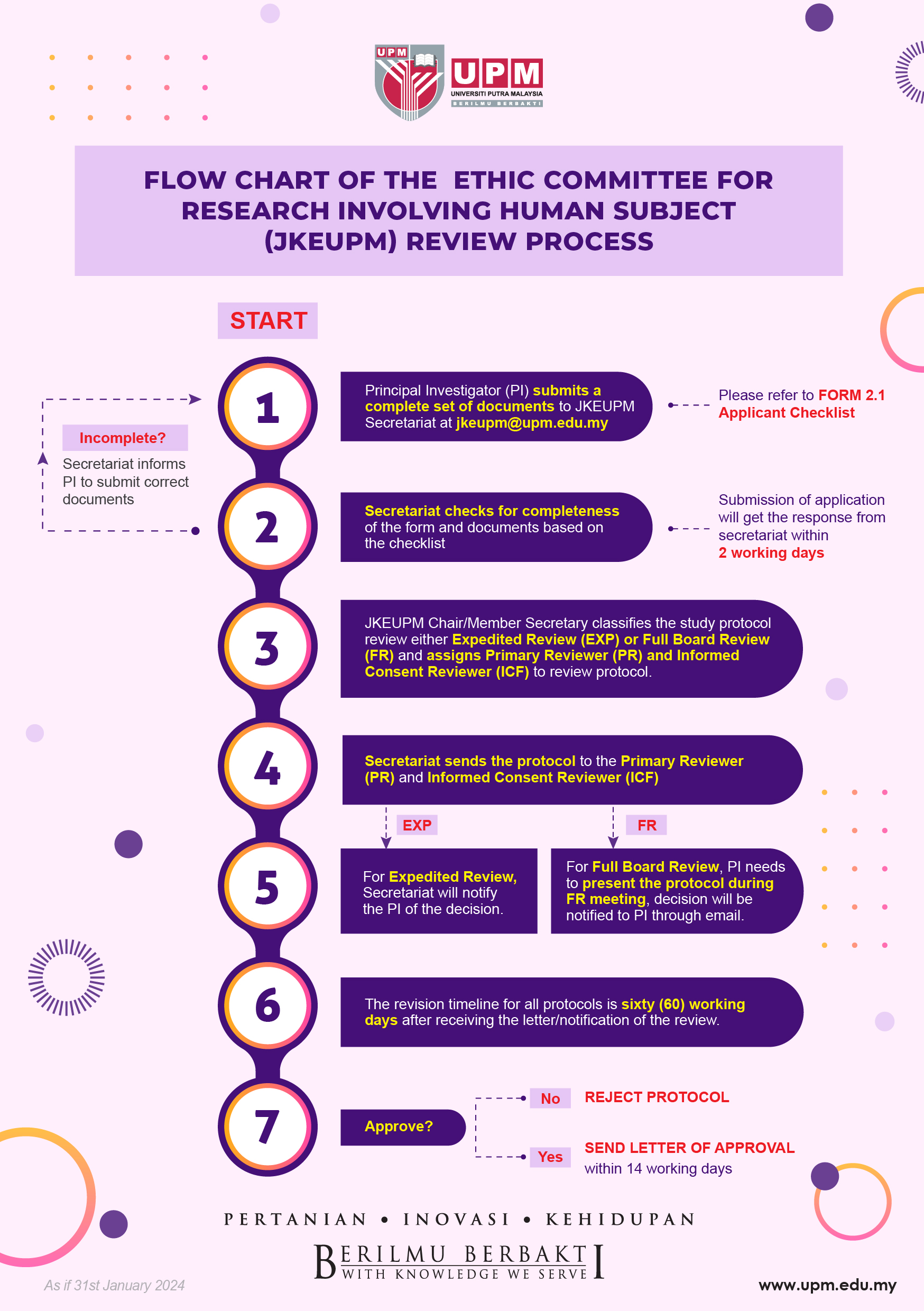
Attached herewith are the Flow Chart of the JKEUPM review process.
For more information please contact+603-9769 1432/ 1438/ 1244/ 1246/ 1602 jkeupm@upm.edu.my
Updated:: 13/02/2024 [maisarahnoor]
MEDIA SHARING